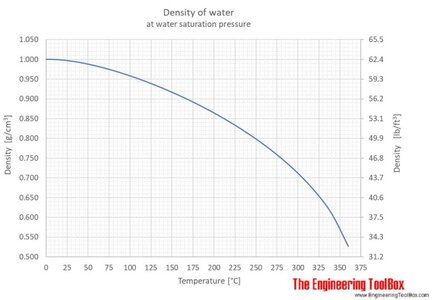
Water is at it's most dense right before it freezes.
When it freezes, it forms crystals that are less dense, so ice floats. The increase in density of the water would be more observable than the change in viscosity. Increased density means more buoyancy, more thrust and other observed phenomenon. Similarly the air is more dense when colder so more oxygen atoms per unit volume making motor run slightly leaner.
When it freezes, it forms crystals that are less dense, so ice floats. The increase in density of the water would be more observable than the change in viscosity. Increased density means more buoyancy, more thrust and other observed phenomenon. Similarly the air is more dense when colder so more oxygen atoms per unit volume making motor run slightly leaner.