- Location
- Chattanooga, TN
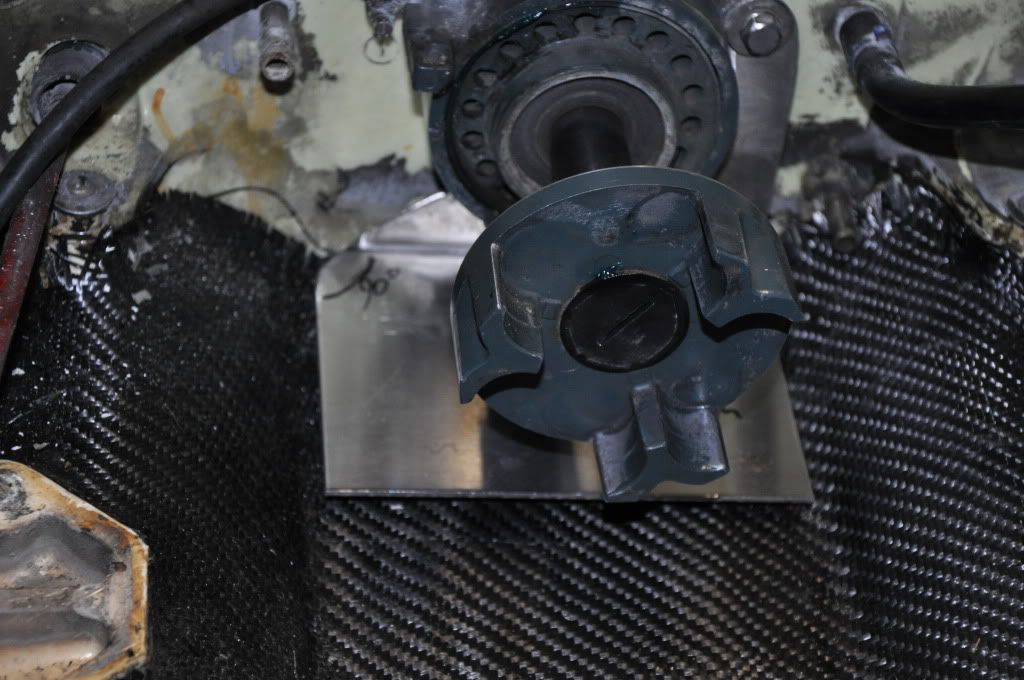
So i didnt want to spend 50+ on my bilge bracket so i made one. Now the way it sits now i have it at just under 1/2 inch off the bottom, i can probably get it a little closer. Just wondering how far off the hull should i have it sitting?



Last edited:
